Abstract
Introduction Sickle cell disease (SCD) is caused by pathologic variants in both alleles of the β-globin gene. Elevated fetal hemoglobin (HbF) levels inhibit sickle cell formation and ameliorate symptoms and improve survival in patients with SCD (Hebert, Am J Hematol, 2020;95:1235-45). BIVV003 is a novel therapeutic product comprising autologous CD34+ hematopoietic stem precursor cells (HSPC) modified ex vivo by zinc finger nucleases (ZFN) targeting specifically the BCL11A gene erythroid-specific enhancer (ESE) to increase endogenous HbF production in erythrocytes (RBC).
Methods PRECIZN-1 (NCT03653247) is an ongoing first-in-human, open label, single arm, multi-center study evaluating safety and tolerability of BIVV003 (n=8; aged 18-40 years) with severe SCD. Eligible subjects underwent mobilization and apheresis with plerixafor 240 ug/kg/day for up to 3 days to collect autologous CD34+HSPCs for manufacturing of BIVV003. HSPCs were transfected ex vivo with ZFN mRNAs targeting the ESE region of the BCL11A locus to manufacture BIVV003. After myeloablative conditioning with busulfan 3.2 mg/kg/day intravenously (IV) for 4 days with dose adjustment based on pharmacokinetics, subjects received BIVV003 as a single minimum dose of 3× 106 CD34+ HSPC/kg IV. Subjects were monitored for stem cell engraftment and hematopoietic recovery, adverse events (AEs), clinical and laboratory hemolysis markers, total hemoglobin (Hb) and HbF, RBC containing HbF (F cells), and SCD-related events for up to 104 weeks post-BIVV003 infusion.
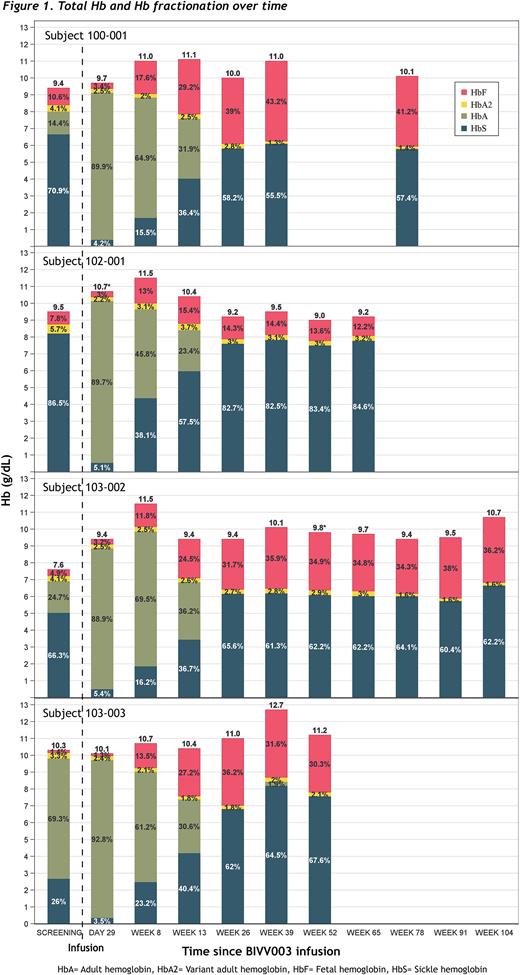
Results As of the cutoff date of 03 May 2022, 4 subjects have received BIVV003 infusions up to 125 weeks of follow-up. A fifth subject was dosed with BIVV003 on 04 May 2022 and has engrafted. Among the 5 subjects, the male:female ratio is 3:2, the age range is 18-35 years, and 4 have genotype HbSS and 1 HbS-β0. No subject has required HSPC rescue; all subjects had hematopoietic reconstitution without requiring RBC or platelet transfusions for more than one month post-BIVV003 infusion. The average time to neutrophil and platelet engraftment was 23 days and 33 days, respectively. In bone marrow aspirates 26 weeks post-infusion, the frequency of cells containing indels at the BCL11A ESE target locus was in the range of 17.1-34.1%. No clonal dominance has been observed in bone marrow or peripheral blood cells. By Week 26, all 4 subjects reached a protective level of ≥ 10 pg HbF/F cell that inhibits HbS polymerization (Steinberg, Blood, 2014;123:481-485), and this level was sustained in 3 out of 4 subjects through the most recent assessment at Week 104, 78, and 52, respectively, along with F cell levels of 90% or higher. The change in total Hb from baseline (at screening) to the latest visit was -0.3-3.1 g/dL, and the HbF fraction increased to 12.2-41.2% (Figure 1). Three subjects have had no recurrence of severe vaso-occlusive crises (VOCs) post-infusion, compared to experiencing 6 to 22 severe VOCs in the 2 years pre-study. The one subject who did not sustain a HbF/F level ≥ 10 pg experienced 2 severe VOCs at 9 and 16 months post-infusion. The adverse events (AEs) reported were consistent with plerixafor mobilization and busulfan myeloablative conditioning. No AEs related to BIVV003 were reported.
Conclusions The interim safety and efficacy results reported here confirm the potential therapeutic value of ZFN-mediated modification of the BCL11A ESE region and BIVV003 infusion to address current unmet needs of patients with SCD. BIVV003 has been well tolerated in the 5 subjects infused to date, with no AEs related to BIVV003. Three of the 4 subjects infused and with over 52 weeks of follow-up have had stable engraftment of ZFN-modified HSPCs resulting in sustained elevated HbF levels and absence of severe VOCs post-BIVV003 administration. A fifth subject received BIVV003 manufactured using an improved process that has shown in internal experiments to increase the number of ZFN-modified long-term progenitors in the drug product.
Disclosures
Abedi:Celgene: Consultancy, Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; AbbVie: Speakers Bureau; Kite, a Gilead Company: Speakers Bureau; Orca Bio: Research Funding; CytoDyn: Current equity holder in publicly-traded company. Boismenu:Sangamo Therapeutics: Current Employment, Current equity holder in publicly-traded company; Coherus Biosciences: Current equity holder in publicly-traded company; Merrimack Pharmaceuticals: Current equity holder in publicly-traded company; IgGenix: Current equity holder in private company. Chen:Sangamo Therapeutics: Current Employment. Hsu:Sangamo Therapeutics: Current Employment, Current equity holder in publicly-traded company. Cockroft:Annexon Biosciences: Current equity holder in publicly-traded company; Sangamo Therapeutics: Current Employment, Current equity holder in publicly-traded company. Galeon:Sanofi: Current Employment, Current equity holder in publicly-traded company. Rendo:Sanofi: Current Employment, Current equity holder in private company. Walters:Vertex Pharmaceuticals: Consultancy; Ensoma, Inc.: Consultancy; AllCells, Inc.: Consultancy; BioLabs, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal